
OPTIMIZATION OF A PHOTOCATALYTIC DECOLORIZATION PROCESS BY USING GENETIC ALGORITHMS
Abstract
This paper proposes a genetic algorithm and neural network based procedure for estimation the optimal conditions for a dyestaff wastewater treatment process consisting of a heterogeneous photocatalytic oxidation.
A simulated dyestaff effluent containing the azo dye Reactive Black 5 (RB5) is decolorized by a photocatalytic reaction using TiO2 P-25 as catalyst in the presence of Fe+3 and H2O2. The 16 series of experimental data obtained in different conditions constitute the training and validation data sets for neural network modeling. A simple feed forward neural network with two hidden layers was projected and used to predict evolution in time of the decolorization of this type of wastewater.
In the second stage, the neural model was included in the optimization procedure solved with a simple genetic algorithm. The goal of the optimizations is to calculate the optimal reaction conditions (illumination time and amounts of reagents) which assure an imposed value for the transmittance.
Keywords: neural network modeling, genetic algorithm optimization, wastewater, photocatalysis, RB5, dye.
Introduction
The elimination of toxic chemicals from wastewater is presently one of the most important subjects in pollution control. These pollutants may originate from industrial applications (petroleum refining, textile processing etc.) or from household and personal care areas (pesticides and fertilizers, detergents etc.); several of them are resistant to conventional chemical and biological treatment methods. The search for effective means of removing these compounds is of interest to regulating authorities everywhere.
The textile dyeing and finishing industry is one of the major pollutants among the industrial sector, since modern dyes are characterized by great stability and a large degree of aromatics in their structure. Almost 30 % of the amount of dyes used is released in the wastewater, so these are highly coloured and with a great organic matter (Easton, 1995; Forgacs et al., 2004). The nonbiodegradability of textile wastewater is due to their high content of dyestuffs, surfactants and other additives. Due to the stability of modern dyes, conventional biological treatment methods for industrial wastewater are ineffective, frequently resulting in an intense coloured discharge from the treatment facilities. Additionally, they are readily reduced under anaerobic conditions to potentially hazardous aromatics. Thus, there is a need for developing more effective treatment methods in eliminating dyes from a waste stream at its source.
Heterogeneous and homogeneous solar photocatalytic detoxification methods (TiO2/H2O2, Fe+3/H2O2) have shown recently great promise for the treatment of industrial wastewater, groundwater and contaminated air. Additionally, the semiconductor mediated photocatalytic process has shown also great potential for disinfection of air and water, thus making possible a number of applications (Peral et al., 1997; Alfano et al., 2000; Malato et al., 2003).
General description of heterogeneous and homogeneous photocatalysis under artificial or solar irradiation is presented in several excellent review articles (Serpone et al., 2002; Hoffman et al., 1995; Fujishima et al., 2000; Herrmann et al., 1999). A brief summary is presented here only for the sake of completeness.
It is well established that by the irradiation
of an aqueous TiO2 suspension with light energy greater than the
band gap energy of the semiconductor (Eg > 3.2 eV), conduction band
electrons (e-) and valence band holes (h+) are generated.
Part of the photogenerated carriers recombine in the bulk of the semiconductor,
while the rest reach the surface, where the holes, as well as the electrons,
act as powerful oxidants and reductants, respectively. The photogenerated electrons react with the
adsorbed molecular O2 on the Ti(III)-sites, reducing it to
superoxide radical anion O2.-, while the photogenerated
holes can oxidize either the organic molecules directly, or the
Although it is well known for some time that the Fenton reagent, a mixture of Fe+2 salts and H2O2, can easily oxidize organic compounds, it has been applied for water and soil treatment only during the last years (Chamarro et al., 2001; Bidga, 1995; Lee et al., 2001). This reagent is an attractive oxidative system, which produces in a very simple way OH. radicals (eq. (1)) for wastewater treatment, due to the fact that iron is a very abundant and non toxic element and hydrogen peroxide is easy to handle and environmentally safe. Furthermore it was found that the reaction can be enhanced by UV/VIS light (artificial or natural), producing additional OH. radicals and leading to the regeneration of the catalyst (eq. (2)) (Photo-Fenton reaction) (Oliveros et al., 1997; Fallmann et al., 1999; Arana et al., 2001).
Fe+2 + H2O2![]() Fe+3 + OH- + OH.
Fe+3 + OH- + OH.
Fe+3 + H2O + hv
(<450 nm) ![]() Fe+2 + H+ + OH.
Fe+2 + H+ + OH.
These reactions are known to be the primary forces of the photochemical self-cleaning of atmospheric and aquatic environment (Faust et al., 1993).
The photocatalytic degradation of azo dyes containing different functionalities has been reviewed using TiO2 or the Photo-Fenton reagent as photocatalysts in aqueous solutions under solar and UV-A irradiation (Muruganandham et al., 2006; Augugliaro et al., 2002; Neppolian et al., 2002). The combination of both processes, as has been reported by other authors (Marugan et al., 2006; Mrowetz et al., 2004), leads to an enhancement of the removal rate of the pollutants due to the fact that Fe+3 ions and H2O2 act as scanvengers of the electrons, which are photogenerated in the conduction band of TiO2, while the produced Fe+2 ions can participate again in the Fenton reaction (eq. 1).
The phenomenological treatment of such photochemical systems are very complex. In general, the rate of reaction in heterogeneous photocatalytic systems is a complex nonlinear function of catalyst loading, light intensity, initial solution pH, reactant and oxidants concentration, etc. Due to these reasons, the ability of systems such as neural networks (NN) to recognize and reproduce cause-effect relationships through training, for multiple input-output systems, has gained recently popularity, in various areas of chemical engineering (Ozkaya et al., 2007; Torrecilla et al., 2007), also in the field of photocatalytic treatment of wastewater (Pareek et al., 2002; Duran et al., 2006; Toma et al., 2004; Salari et al., 2005).
The solution of an optimisation problem can be found through, among others, deterministic or stochastic approaches. The former composes the traditional optimisation methods (direct and gradient-based methods) and have the disadvantages of requiring the first and/or second-order derivatives of the objective function and/or constraints or of being not efficient in non-differentiable or discontinuous problems. Furthermore, the deterministic methods are dependent on the chosen initial solution and can tend to converge towards local extrema of the fitness function, which is clearly unsatisfactory for problems where the fitness varies non-monotonously with the parameters. The stochastic methods, such as genetic algorithms (GA), do not possess these drawbacks. GAs are part of the so-called evolutionary algorithms and compose a search and optimisation tool with increasing application in scientific problems. They do not need to have any information about the search space, just needing an objective/fitness function that assigns a value to any solution (Deb, 1999).
In recent years, due to the rapid progress in computing technology available, there is a growing interest in optimization techniques based on genetic and evolutionary algorithms. The basic algorithm, simple GA or SGA, offers advantages over more traditional optimization approaches (e.g. several search techniques, Pontryagin's principle, SQP etc.), in some cases. Moreover, it has the advantages that it does not require good initial guesses for the values of the decision variables. It uses a population of several points simultaneously along with probabilistic operators (reproduction, crossover and mutation), inspired by natural genetics. Additionally, SGA has the advantage that it uses only the values of the objective functions and not any derivatives, as required by gradient search techniques (Guria et al., 2005).
Because of their flexibility, ease of operation, minimal requirements and global perspective, these algorithms have been successfully used in a wide variety of problems (Deb, 2001) GA has found various applications in chemical engineering including process control, gas pipeline design, pattern recognition of multivariate chemical data, optimization of reaction rate parameters, multipurpose chemical batch plant design, and scheduling (Yan et al., 2003) Details about different types of genetic algorithms - simple genetic algorithm or different adaptations for problems with multiple constraints - and a series of their applications in optimization of the chemical processes has been descried in the reviews of Bhaskar (2000) and Nandasana (2003).
The main goal of this paper is to develop a general procedure based on neural networks and genetic algorithms which could be applied to the complex optimization problems. Neural networks are used as efficient modeling tool and genetic algorithms as solving method of optimization; a neural model of the process is included in the optimization procedure. The case study is the estimation of the evolution in time of a dyestuff wastewater treatment process consisting of a photocatalytic oxidative reaction. It is followed a certain decolorization degree related to optimal working conditions: illumination time, amounts of catalyst TiO2 P-25, H2O2 and Fe+3. The GA optimization procedure has proved easily to apply with useful and accurate results.
Experimental
Reagents
This
synthetic wastewater (CSW) was made according to recipe used for dyeing of
cotton fabrics and was of the following composition
TiO2 P-25 of Degussa
(anatase/rutile = 3.6/1, surface area
Procedures and analysis
Experiments were carried-out in a closed Pyrex cell of 500 ml capacity, provided with ports, at the top, for bubbling air necessary for the reaction to take place. The reaction mixture was maintained as suspension by magnetic stirring. Previously to irradiation, the reaction mixture was left 15 minutes in the dark with the aim at achieving the maximum adsorption of the dye onto the semiconductor catalyst surface. The irradiation was performed with a 9 W central lamp. The spectral response of the irradiation source (Osram Dulux S 9W/78 UV-A) according to the producer is ranged between 340 and 400 nm with a maximum at 366 nm and two additional weak lines in the visible region. The photon flow per unit volume of the incident light was determined by chemical actinometry using potassium ferrioxalate. The initial light flux, under exactly the same conditions as in the photocatalytic experiments, was evaluated to be 1.16 x 10-4 Einstein min-1.
In
all cases, during the experiments, 500 ml of a acidified (pHo=3.2)
dye solution containing the appropriate amounts of semiconducting powder, H2O2
and Fe+3 was magnetically stirred before and during irradiation.
Specific quantities of samples were withdrawn at periodic intervals and
filtered through a 0.45 mm filter (Schleicher and Schuell) in order to remove the catalyst particles. With the aim at
assessing the extent of color removal, changes in the concentration of Reactive
Black 5 were observed from its characteristic absorption band at 585 nm, using
a UV-VIS spectrophotometer Shimadzu UV-
Formulation of the optimization problem
In complex chemical processes, the ability to select optimum operating conditions in the presence of multiple conflicting objectives, given the various constraints of the process, can present a major challenge. The general solution of an optimization problem can be obtained in terms of the following four elements: an accurate model of the process, a selected number of control variables, an objective function and a suitable numerical method for solving the specified optimization problem.
A good process model is a prerequisite for application in the optimal control strategy. The modeling with neural networks has a series of advantages: the possibility to apply this method to complex non-linear processes, the ease in obtaining and using these models, the possibility to substitute experiments with predictions. Neural networks possess the ability to learn what happens in the process without actually modeling the physical and chemical laws that govern the system. Neural models need only input-output data (experimental or simulation data) so, their advantages are evident against the complexity of the computation.
GAs have several characteristics that make them attractive to solve optimization problems where the model is only available in an implicit form (black-box model). Firstly, due to the fact they are based on a direct search method, it is not necessary to have explicit information of the mathematical model or its derivatives. Secondly, the search for the optimal solution is not limited to one point but it rather relies on several points simultaneously, therefore the knowledge of initial feasible points is not required and such points do not influence the final solution (Leboreiro et al., 2004).
A series of experiments have been carried out for the decolorization of
the synthetic dyestaff effluent containing the Reactive Black 5 (RB5) as a
model azo dye, by photocatalytic degradation using TiO2 P-25 as catalyst in the presence of Fe+3
and H2O2. Based on these experimental data, a neural
modeling methodology was developed to describe the dependence of the water
decolorization process of different reaction conditions and irradiation time. A MLP(4:9:3:1) - feed-forward
neural network with 4 variables as inputs (illumination time in min, amount of
catalyst TiO2 P-
Figure 1
The feed-forward, multilayered neural network is the most used network's type because the simplicity of its theory, ease of programming, good results and for its universal function in the sense that if topology of the network is allowed to vary freely it can take the shape of any broken curve.
The model MLP (4:9:3:1) was tested against training and validation data. Good agreement between experimental data and neural network predictions (average relative errors less than 1.5 % and correlation over 0.99) proves that the neural model is convenient for the optimization procedure. Details concerning the development and testing this model were given in a previous paper (Suditu et al., 2007).
In this study, the optimization problem includes the neural model which is represented as:
NN [Inputs: t, TiO2, H2O2, Fe+3 ; Output: D] (3)
The vector of control variables , u, has the components:
u = [t, TiO2, H2O2, Fe+3] (4)
An admissible control input u* should be formed in such a way that the performance index, J, defined by the following equation, are minimized:
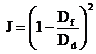 (5)
(5)
subject to:
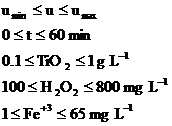 (6)
(6)
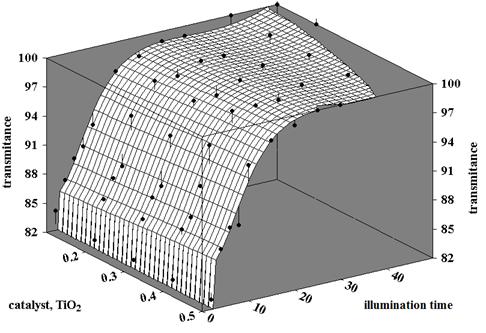
with t representing illumination time, Df - transmittance at the end of the process and Dd - an imposed value for this parameter.
The transmittance is a measure of the dye elimination from wastewater, with values between 0 and 100 %. High values for this parameter mean high degree of dye elimination.
The constraints are very important to define the range of variation of parameters and to disregard possible solutions that could be interesting in a theoretical approach to the problem.
In other words, the optimization problem to be solved can be formulated as: Which are the optimal working conditions (time, amount of catalyst TiO2 P-25, amounts of H2O2 and Fe+3) necessary to obtain an imposed transmittance under the given experimental conditions?
The optimization procedure includes a neural model and it is solved with a simple genetic algorithm. The fitness function of the GA is the scalar objective function (5). Figure 2 illustrates this optimization procedure. Genetic algorithm provides, after an iterative calculus, the optimal values for decision variables (time, TiO2, H2O2, Fe+3), which are the inputs for the neural network model. With these inputs, the neural network computes the final value of transmittance, Df which will be compared with the desired value, Dd. If the two values are identical or there is a very tight difference between them, we can conclude that the task of the optimization, represented by minimum of the objective function, J, is achieved.
Figure 2
GA optimization technique
In our GA model, we used real values encoding for the chromosomes. There are other approaches for genetic algorithm based optimization which use binary solution representation, as it is the simplest type of encoding, in which chromosomes are composed only of 1's and 0's. Even the number of alleles is thus rather small (two), this encoding is very common, because it is very easy to use. However, value encoding is more general, because genes are real numbers. Some experiments (Michalewicz, 1996) have shown that real value encoding is more time efficient, with better precision of the solutions.
There are different methods for selection phase; our paper uses rank selection which first ranks the population and then every chromosome receives fitness from this ranking. The worst will have fitness 1, second worst 2 etc. and the best will have fitness n (number of chromosomes in population).
The probability that the individual i should be chosen in the rank selection is:
![]() (7)
(7)
The recombination (crossover) has as main purpose the recombination the features of two randomly selected parents from the mating pool with the aim of producing better offspring.
Arithmetic real value crossover produces a linear combination
of the parents. Given a uniform random number ![]() ,
,
![]() (8)
(8)
or
![]() (9)
(9)
where C is the real value chromosome of the child, and M and F are the chromosomes of the parents.
The variant of crossover used in this study supposes different points for all genes, that means the new individual will no longer be on the line segment that links its parents. The r value is different for every gene. The offspring will look more like one parent regarding a feature and less regarding another.
After recombination, offspring undergoes to mutation. Generally, the mutation refers to the creation of a new chromosome from one and only one individual with predefined probability. Mutation is used to produce small perturbations on chromosomes to promote diversity of the population.
Our GA includes a variant of mutation named resetting. A gene value is reset to a random value in its search interval. The purpose is to refresh the search process, in case when the genetic diversity of the population decreases (so no longer converges to the solution) or the algorithm has converged into a local optimum. Each gene is independently considered, and mutation gives it a new random value in the initialization interval. Only some genes change (possibly all, but unlikely).
After the three operators are carried, the offspring is inserted into the population, replacing the parent chromosomes in which they were derived from, producing a new generation. The best individual is copied directly into the new population (the elitism technique) and the rest of the individuals are replaced by the new generations. So, in order to keep the best solution, we have considered an elitism factor fe = 1, that is the best individual is copied directly in the new generation. That ensures that the overall solution of the GA will not get worse.
Population size, number of generations, crossover probability and mutation probability are known as the control parameters of genetic algorithms. The values of these parameters must be specified before the execution of GA and they depend on the nature of the objective function. One important thing in using GA as solving method is to adjust its parameters to the particular problem approached so as to obtain good solutions and to preserve the algorithm from a preliminary convergence.
There is no general termination criteria for GA. Predetermined number of generations, time or comparison of the best solutions to average fitness may be taken as stopping criterion. In our work, the number of generations is established before running and constitutes the stop criterion.
Several tests lead to the following values: pop_size = 50 (size of initial population), max_gen = 100 (maximum number of generations), cross_rate = 0.9 (rate of crossover) and mut_rate = 0.03 (rate of mutation).
The fitness function in GA is the objective function of the optimization procedure. The results of the optimization are represented by the values of the decision variables (illumination time, amounts of catalyst, H2O2 and Fe+3) that lead to a minimum value of the objective function, which means the achievement of the imposed value for the transmittance.
The optimization procedure is implemented in Matlab 7.0 with original software, as specific functions were programmed for each phase of the genetic algorithm.
The optimization procedure based on a simple genetic algorithm and a neural network model applied in this paper is easy to manipulate and provides accurate results. In this way, a theoretical complete analysis of the decolorization process approached here is performed, with useful information for the practical applications.
Conclusions
This paper provides a general and simple optimization methodology, based on genetic algorithms and neural networks, applied to a wastewater decolorization process. The genetic algorithm solves the optimization problem and the neural network constitutes the model included in the optimization procedure.
Simple architecture of the neural network is proposed for process modeling: feed forward neural network with two hidden layers. The simple genetic algorithm proves to be a good tool for solving the optimization problem, providing important information useful in experimental practice.
The method can be easily extended and adapted to other environmental oriented processes, with high chances of providing accurate results by simple handling.
References
Alfano, O., Bahnemann, D., Cassano, A., Dillert, R., Goslich, R. (2000). Photocatalysis in water environments using artificial and solar light, Catal. Today, 58, 199-230.
Arana, J., Rendon, E.T., Rodriguez, J.M.D., Melian, J.A., Diaz, O., Pena, J. (2001). Highly concentrated phenolic wastewater treatment by the Photo-Fenton reaction, mechanism study by FTIR-ATR, Chemosphere, 44, 1017-1023.
Augugliaro, V., Baiocchi, C., Prevot, A., López, E., Loddo, V., Malato, M., Marcí, G., Palmisano, L., Pazzi, M., Pramauro, E. (2002). Azo-dyes photocatalytic degradation in aqueous suspension of TiO2 under solar irradiation, Chemosphere, 49, 1223-1230.
Bidga, R.J. (1995). Consider Fenton chemistry for
wastewater treatment, Chem.
Bhaskar, V., Gupta, S.K., Ray, A.K. (2000). Applications of multiobjective optimization in chemical engineering, Reviews in Chemical Engineering, 16, 1-54.
Chamarro, E., Marco, A., Esplugas, S. (2001). Use of Fenton reagent to improve organic chemical biodegradability, Water Res., 35,1047-1051.
Deb, K. (1999). An introduction to Genetic Algorithms, in Sadhana-Academy Proceedings in Engineering Sciences, 24, 293-315.
Deb, K. (2001).
Multiobjective optimization using evolutionary algorithms.
Duran, A., Monteagudo, J.M., Mohedano, M. (2006). Neural networks simulation of photo-Fenton degradation of Reactive Blue 4, Applied Catalysis B: Environmental, 65, 127-134.
Fallmann, H., Krutzler, T., Bauer, R., Malato, S., Blanco, J. (1999). Applicability of the Photo-Fenton method for treating water containing pesticides, Catal. Today 54, 309-319.
Faust, B.C., Zepp, R.G. (1993). Photochemistry of aqueous Iron (III) polycarboxylate complexes: roles in the chemistry of atmospheric and surface waters, Environ. Sci. Technol., 27, 2517-2522.
Forgacs, E., Cserhati, T., Oros, G. (2004). Removal of synthetic dyes from wastewaters: a review, Environment International, 30, 953- 971.
Fujishima, A., Rao, T., Tryk, D. (2000). Titanium dioxide photocatalysis, Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 1, 1-21.
Herrmann, J.M. (1999). Heterogeneous Photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants, Catal. Today, 53, 115-29.
Hoffman, M., Martin, S., Choi, W., Bahnemann, D. (1995). Environmental applications of semiconductor photocatalysis, Chem. Rev., 95, 69-96.
Leboreiro, J., Acevedo, J. (2004). Processes synthesis and design of distillation sequence using modular simulators: a genetic algorithm framework, Computers and Chemical Engineering, 28, 1223-1236.
Lee, B.D., Hosomi, M. (2001). Fenton oxidation of ethanol washed distillation-concentrated benzo(a)pyrene: reaction product identification and biodegradability, Water Res., 35, 2314-2319.
Malato, S., Blanco, J., Vidal, V., Alarcon, D., Maldonado, M., Caceres, J., Gernjak, W. (2003). Applied studies in solar photocatalytic detoxification: an overview. Solar Energy, 75, 329-336.
Marugan, J., Lopez-Munoz, M.J., Gernjak, W., Malato, S. (2006). Fe/TiO2/pH interactions in solar degradation of imidacloprid with TiO2/SiO2 photocatalysts at pilot-plant scale, Ind. Eng. Chem. Res., 45, 8900-8908.
Michalewicz, Z. (1996).
Genetic Algorithms + Data Structures = Evolution Programs, Springer Verlag,
Mrowetz, M., Selli, E. (2004). Effects of iron species in the photocatalytic degradation of an azo dye in TiO2 aqueous suspensions, J. Photochem. Photob.A: Chemistry, 162, 89-95
Muruganandham, M., Sobana, N., Swaminathan, M. (2006). Solar assisted photocatalytic and photochemical degradation of Reactive Black 5, J. Hazard. Mat., 137, 1371-1376.
Nandasana, A.D., Ray, A.K., Gupta, S.K. (2003). Applications of the Non-Dominated Sorting Genetic Algorithm (NSGA) in Chemical Reaction Engineering, International Journal of Chemical Reactor Engineering,
Neppolian, B., Choi, H.C., Sakthivel, S., Arabindoo, B., Murugesan, V. (2002). Solar/UV-induced photocatalytic degradation of three commercial textile dyes, J. Hazard. Mat., 89, 303-317.
Oliveros, E., Legrini, O., Hohl, M., Mueller, T.,
Braun, A. (1997). Industrial wastewater treatment: large scale
development of a light-enhanced Fenton
reaction, Chem.
Ozkaya, B., Demir, A., Sinan Bilgili, M. (2007). Neural network prediction model for the methane fraction in biogas from field-scale landfill bioreactors, Environmental Modelling & Software,
Pareek, V.K., Brungs, M.P., Adesina, A.A., Sharma, R. (2002). Artificial neural network modeling of a multiphase photodegradation system, Journal of Photochemistry and Photobiology A: Chemistry, 149, 139-146.
Peral, J., Domenech, X., Ollis, D. (1997). Heterogeneous photocatalysis for purification, decontamination and deodorization of air, J. Chem. Technol. Biotechnol, 70, 117-140.
Salari, D., Daneshvar, N., Aghazadeh, F., Khataee, A.R. (2005). Application of artificial neural networks for modelling of the treatment of wastewater contaminated with methyl tert-butyl ether (MTBE) by UV/H2O2 process, Journal of Hazardous Materials, 125, 205-210.
Serpone, N., Emeline, A.V. (2002). Suggested terms and definitions in photocatalysis and radiolysis, Int. J. Photoenergy,
Suditu, G.D., Secula, M.
S., Piuleac, C.G., Curteanu, S., Poulios,
Toma, F.L., Guessasma, S., Klein, D., Montavon, G., Bertrand, G., Coddet, C. (2004). Neural computation to predict TiO2 photocatalytic efficiency for nitrogen oxides removal, Journal of Photochemistry and Photobiology A: Chemistry, 165, 91-96.
Torrecilla, J., Mena, M., Yáñez-Sedeño, P., García, J. (2007). Application of artificial neural network to the determination of phenolic compounds in olive oil mill wastewater, Journal of Food Engineering, .
Wang, L., Zhang, L., Zheng D.Z. (2006). An effective hybrid genetic algorithm for flow shop scheduling with limited buffers, Computers & Operations Research, 33, 2969-2971.
Yan, X.F., Chen, D.Z., Hu, S.X., 2003. Chaos-genetic algorithms for optimizing the operating conditions based on RBF-PLS model, Computers and Chemical Engineering, 27, 1393-1404.
Figure Captions
Figure 1. Topology of the neural network model, MLP (4:9:3:1).
Figure 2. The optimization method based on NN and GA.
and illumination time and amount of catalyst, for what Df = Dd.
|
Dd |
Time [min] |
TiO2 [g L-1] |
H2O2 mg L-1 |
Fe+3 mg L-1 |
Df |
J |
|
|
||||||
|
Dd |
Time [min] |
TiO2 [g L-1] |
H2O2 mg L-1 |
Fe+3 mg L-1 |
Df |
J |
|
| ||||||
|
Dd [%] |
Time [min] |
TiO2 [g L-1] |
H2O2 [mg L-1] |
Fe+3 [mg L-1] |
c/c0 |
Energy, [kJ] |
|
99 |
20.04 |
0.30 |
453.32 |
50.08 |
0.00 |
10.82 |
|
100 |
19.23 |
0.51 |
517.31 |
50.87 |
0.00 |
10.38 |
|
98 |
14.94 |
0.32 |
363.10 |
52.65 |
0.01 |
8.07 |
|
97 |
14.1 |
0.59 |
411.16 |
17.21 |
0.01 |
7.61 |
|
96 |
11.73 |
0.69 |
321.60 |
19.81 |
0.01 |
6.33 |
|
95 |
10.68 |
0.21 |
273.60 |
34.94 |
0.02 |
5.77 |
|
89 |
9.64 |
0.65 |
505.92 |
2.73 |
0.04 |
5.21 |
|
94 |
8.04 |
0.35 |
299.41 |
39.99 |
0.02 |
4.34 |
|
91 |
8.03 |
0.43 |
469.03 |
9.14 |
0.03 |
4.34 |
|
90 |
7.82 |
0.51 |
397.44 |
10.36 |
0.04 |
4.22 |
|
86 |
7.04 |
0.19 |
306.15 |
41.37 |
0.05 |
3.80 |
|
87 |
6.82 |
0.55 |
474.79 |
12.13 |
0.05 |
3.68 |
|
79 |
6.61 |
0.69 |
408.97 |
7.23 |
0.08 |
3.57 |
|
84 |
6.6 |
0.63 |
299.41 |
10.04 |
0.06 |
3.56 |
|
85 |
6.37 |
0.39 |
222.07 |
43.08 |
0.06 |
3.44 |
|
93 |
5.86 |
0.37 |
398.20 |
45.51 |
0.03 |
3.16 |
|
88 |
5.31 |
0.48 |
250.71 |
41.69 |
0.05 |
2.87 |
|
82 |
5.12 |
0.75 |
275.49 |
16.72 |
0.07 |
2.76 |
|
76 |
5.1 |
0.62 |
495.93 |
13.72 |
0.10 |
2.75 |
|
77 |
4.98 |
0.57 |
549.10 |
13.67 |
0.09 |
2.69 |
|
92 |
4.73 |
0.61 |
297.71 |
30.68 |
0.03 |
2.55 |
|
78 |
4.65 |
0.32 |
317.52 |
47.05 |
0.09 |
2.51 |
|
81 |
4.57 |
0.58 |
301.21 |
20.12 |
0.07 |
2.47 |
|
75 |
4.16 |
0.51 |
434.18 |
18.86 |
0.10 |
2.25 |
|
83 |
3.99 |
0.71 |
267.29 |
25.51 |
0.07 |
2.15 |
|
80 |
3.83 |
0.49 |
282.23 |
40.28 |
0.08 |
2.07 |
|
Dd [%] |
Time [min] |
TiO2 [g L-1] |
H2O2 [mg L-1] |
Fe+3 [mg L-1] |
c/c0 |
Energy, [kJ] |
|
99 |
21.8 |
0.19 |
482.33 |
45.48 |
0.00 |
11.78 |
|
100 |
19.7 |
0.24 |
331.97 |
23.09 |
0.00 |
10.65 |
|
98 |
16.0 |
0.18 |
431.60 |
50.45 |
0.01 |
8.63 |
|
95 |
15.3 |
0.18 |
216.69 |
43.40 |
0.02 |
8.28 |
|
97 |
14.6 |
0.20 |
347.48 |
45.23 |
0.01 |
7.87 |
|
96 |
14.2 |
0.22 |
291.98 |
44.23 |
0.01 |
7.64 |
|
92 |
11.9 |
0.15 |
169.48 |
41.50 |
0.03 |
6.41 |
|
94 |
11.6 |
0.24 |
209.16 |
49.97 |
0.02 |
6.25 |
|
87 |
9.9 |
0.16 |
135.39 |
42.22 |
0.05 |
5.34 |
|
93 |
9.8 |
0.17 |
257.71 |
47.52 |
0.03 |
5.31 |
|
88 |
8.9 |
0.18 |
220.23 |
37.69 |
0.05 |
4.80 |
|
90 |
8.5 |
0.18 |
282.19 |
41.32 |
0.04 |
4.61 |
|
91 |
8.0 |
0.23 |
277.01 |
35.77 |
0.03 |
4.32 |
|
84 |
7.1 |
0.15 |
307.04 |
37.34 |
0.06 |
3.83 |
|
75 |
6.9 |
0.14 |
261.69 |
39.61 |
0.10 |
3.72 |
|
85 |
6.5 |
0.24 |
304.52 |
41.61 |
0.06 |
3.53 |
|
79 |
6.4 |
0.17 |
242.74 |
32.81 |
0.08 |
3.46 |
|
89 |
6.3 |
0.18 |
389.72 |
40.83 |
0.04 |
3.42 |
|
80 |
6.2 |
0.46 |
471.83 |
9.13 |
0.08 |
3.32 |
|
81 |
5.9 |
0.20 |
333.19 |
39.75 |
0.07 |
3.19 |
|
86 |
5.6 |
0.18 |
233.49 |
23.89 |
0.05 |
3.03 |
|
76 |
5.4 |
0.17 |
348.38 |
36.36 |
0.10 |
2.90 |
|
82 |
4.8 |
0.22 |
387.97 |
45.40 |
0.07 |
2.58 |
|
83 |
4.1 |
0.24 |
644.01 |
40.44 |
0.07 |
2.19 |
|
78 |
3.5 |
0.22 |
441.71 |
31.76 |
0.09 |
1.90 |
|
77 |
2.6 |
0.26 |
593.30 |
53.27 |
0.09 |
1.41 |
|
Dd [%] |
TiO2 [g L-1] |
Time [min] |
H2O2 [mg L-1] |
Fe+3 [mg L-1] |
Df [%] |
|
Dd [%] |
TiO2 [g L-1] |
Time [min] |
H2O2 [mg L-1] |
Fe+3 [mg L-1] |
Df [%] |
|
80 |
0.1 |
2 |
532.56 |
58.32 |
80 |
|
88 |
0.1 |
2 |
637.97 |
64.06 |
88 |
|
85 |
0.3 |
2 |
542.88 |
63.17 |
85 |
|
87 |
0.3 |
2 |
560.31 |
64.84 |
87 |
|
85 |
0.5 |
2 |
549.44 |
63.39 |
85 |
|
87 |
0.5 |
2 |
495.51 |
64.71 |
87 |
|
89 |
0.2 |
4 |
605.75 |
50.93 |
89 |
|
94 |
0.2 |
4 |
642.17 |
25 |
94 |
|
90 |
0.4 |
4 |
436.65 |
53.39 |
90 |
|
95 |
0.4 |
4 |
618.43 |
35.01 |
95 |
|
90 |
0.1 |
6 |
374.25 |
18.75 |
90 |
|
96 |
0.1 |
6 |
542.85 |
29.04 |
96 |
|
89 |
0.3 |
6 |
269.63 |
13.19 |
89 |
|
98 |
0.3 |
6 |
593.03 |
33.31 |
98 |
|
90 |
0.5 |
6 |
235.68 |
44.05 |
90 |
|
99 |
0.5 |
6 |
635.50 |
35.81 |
99 |
|
89 |
0.1 |
8 |
321.73 |
33.73 |
89 |
|
95 |
0.1 |
8 |
450.58 |
44.11 |
95 |
|
93 |
0.2 |
8 |
332.40 |
45.80 |
93 |
|
98 |
0.2 |
8 |
534.75 |
34.03 |
98 |
|
90 |
0.3 |
8 |
473.58 |
3.79 |
90 |
|
96 |
0.3 |
8 |
332.91 |
23.04 |
96 |
|
90 |
0.1 |
10 |
246.30 |
38.62 |
90 |
|
100 |
0.1 |
10 |
744.60 |
24.39 |
100 |
|
95 |
0.3 |
10 |
284.94 |
39.16 |
95 |
|
100 |
0.3 |
10 |
589.82 |
29.37 |
100 |
|
95 |
0.4 |
10 |
295.99 |
7.62 |
95 |
|
100 |
0.4 |
10 |
586.68 |
28.62 |
100 |
|
98 |
0.5 |
10 |
380.65 |
46.88 |
98 |
|
99 |
0.5 |
10 |
691.09 |
45.72 |
99 |
|
Imposed TiO2 [g L-1] |
Imposed illumination time [min] |
Interval for transmittance [%] |
|
0.1 |
2 |
80 88 |
|
0.2 |
2 |
80 88 |
|
0.3 |
2 |
80 87 |
|
0.4 |
2 |
80 86 |
|
0.5 |
2 |
80 87 |
|
0.1 |
4 |
80 95 |
|
0.2 |
4 |
80 95 |
|
0.3 |
4 |
80 95 |
|
0.4 |
4 |
80 96 |
|
0.5 |
4 |
80 97 |
|
0.1 |
6 |
80 98 |
|
0.2 |
6 |
80 99 |
|
0.3 |
6 |
80 100 |
|
0.4 |
6 |
80 100 |
|
0.5 |
6 |
80 100 |
|
0.1 |
8 |
80 100 |
|
0.2 |
8 |
80 100 |
|
0.3 |
8 |
81 100 |
|
0.4 |
8 |
84 100 |
|
0.5 |
8 |
83 100 |
|
0.1 |
10 |
84 100 |
|
0.2 |
10 |
87 100 |
|
0.3 |
10 |
90 100 |
|
0.4 |
10 |
92 100 |
|
0.5 |
10 |
91 100 |
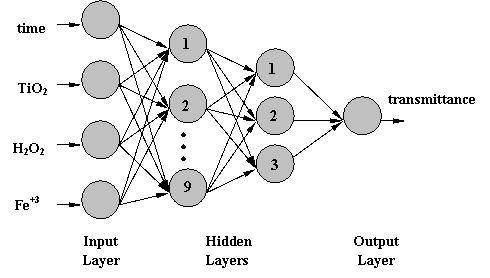
Figure 1
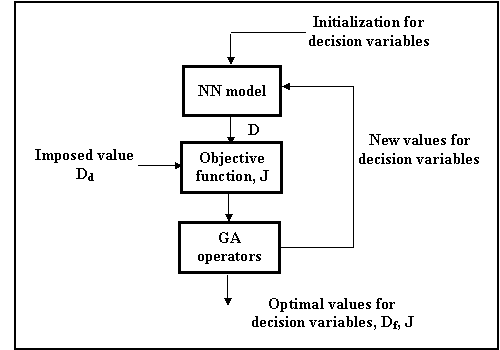
Figure 2
|
Politica de confidentialitate |
| Copyright ©
2025 - Toate drepturile rezervate. Toate documentele au caracter informativ cu scop educational. |
Personaje din literatura |
| Baltagul – caracterizarea personajelor |
| Caracterizare Alexandru Lapusneanul |
| Caracterizarea lui Gavilescu |
| Caracterizarea personajelor negative din basmul |
Tehnica si mecanica |
| Cuplaje - definitii. notatii. exemple. repere istorice. |
| Actionare macara |
| Reprezentarea si cotarea filetelor |
Geografie |
| Turismul pe terra |
| Vulcanii Și mediul |
| Padurile pe terra si industrializarea lemnului |
| Termeni si conditii |
| Contact |
| Creeaza si tu |