One of the benefits of arthroscopy has been the delineation of chondral injuries. Demonstration of articular cartilage involvement in knee injuries has elucidated many of the complaints of and causes for persistent discomfort in the symptomatic patient. Subsequently, the orthopaedist must be familiar with the characteristics of normal articular cartilage injury and the physiologic response.
An excellent description of gross and histologic characteristics of articular cartilage has been presented by Rand.231 To the naked eye, articular cartilage appears as a smooth covering of the joint surface. This is deceptive: articular cartilage, composed of cells (chondrocytes) in an extracellular matrix, is a highly complex anatomic structure. Less than 5% of tissue volume in articular cartilage is composed of cells, less than any other tissue in the body. Articular cartilage is also unique in that no vascular, neural, or lymphatic contribution is involved in its maintenance. Subsequently, nourishment depends on the diffusion of nutrients.219 In addition to this diffusion of nutrients, anaerobic pathways are well developed in chondrocytes to allow for synthesis of the extracellular matrix.
The extracellular matrix contains fluid and structural macromolecules.192 Fluid consists of water and proteins and accounts for 70% of the volume.192,219,231 Its fluid or water content, which is not bound by a membrane, accounts for the mechanical characteristics of articular cartilage. The interaction of the fluid and the extracellular macromolecules allows the matrix to resist compression without permanent deformation.192
Macromolecules, the other portion of the extracellular matrix, are fibrillar and nonfibrillar. Collagen is the fibrillar component and accounts for 50% of the tissue dry weight.192 Type II collagen exists only in articular cartilage, the nucleus pulposus, and the vitreous body.192 The collagen accounts for the overall uniform appearance of the articular cartilage and its tensile strength. The nonfibrillar component of the articular cartilage, the ground substance, is proteins and glycoproteins. The proteoglycans are the major macromolecule of the nonfibrillar component of articular cartilage.192 The dominant types of polysaccharides are chondroitin sulfate, keratin sulfate, and hyaluronic acid.192,219 The proteoglycan unit is the central protein core to which are attached various polysaccharide aggregates. Proteoglycans are responsible for the resilience and stiffness for compressive forces, whereas the collagen component and the extracellular matrix provide tensile stiffness.192 Compressive loads displace the water from the proteoglycan units. Removing the force allows the water to return to the preloaded state: this is why the articular cartilage is strong enough to resist compression and yet resilient enough to minimize permanent deformation. The articular cartilage is 3 to 5 mm thick throughout the knee.208 It seems thicker in a patient with increased body weight.219
Four zones of articular cartilage cover the subchondral bone. Zone 1 is the superficial or tangential layer, zone 2 the transitional layer, zone 3 the deep radial layer, and zone 4 the calcified cartilage layer. The superficial zone is the thinnest layer, with the highest collagen content and subsequently the greatest ability to resist sheer stresses. The transitional zone, composed almost entirely of proteoglycans, is less strongly bound. This zone is the transition between the shearing forces of the surface layer to the compression forces in the cartilage layers. The radial zone is the largest part of the articular cartilage and, while distributing loads, resists compression. The fourth, the zone of calcified cartilage, the tidemark, separates the hyaline cartilage from the subchondral bone. It is believed that the lubrication of the articular cartilage is due to the 'weeping' of the water between the glycoproteins and the collagen fibers.221
A major concern about articular cartilage injury is its potential ability to heal the defect. Although this is controversial, certain facts substantiate the claim. The avascularity of articular cartilage prevents a vascular response when there is cartilage injury alone. When the subchondral bone is also injured, a vascular response might ensue. Chondrocytes in articular cartilage, however, are capable of cell division and DNA synthesis.197 Similarly, the chondrocytes are capable of increased proteoglycan synthesis. Although chondrocytes can synthesize proteoglycans, this extracellular matrix is susceptible to proteolytic enzymes. Leukocytes possess proteolytic enzymes that can degrade articular cartilage, as is seen in septic arthritis. In this situation, repair of the extracellular matrix depends on the extent of damage done by the hydrolytic enzymes. Similarly, response to mechanical injury by the articular cartilage seems to depend on the extent of the injury.
Two types of repair mechanisms exist: extrinsic and intrinsic.231 The intrinsic repair depends on the functioning of the chondrocytes to synthesize a new matrix. Although human studies suggest that articular cartilage is not ordinarily repaired,196,228,231,232,238 animal studies suggest that articular cartilage injuries are capable of healing by intrinsic repair.193,222,231,232 Regardless, the avascular nature of articular cartilage limits the inflammatory response; therefore, any intrinsic attempt depends on the diffusion of nutrients and thus is limited. From a review of the literature,193,194,196,199,201,203,222,224,225,228,231,232,233,238 it appears that superficial cartilage lacerations will not heal by an intrinsic response, but they do not seem to lead to osteoarthritic changes.
Extrinsic repair depends on either a synovial reaction or subchondral bone being affected at the time of injury. When the bone is responding to the injury, it forms a fibrin clot, which is subsequently replaced by granulation tissue and then fibrous tissue.218 The reparative tissue is almost always fibrocartilaginous, although some animal species have displayed healing of full-thickness defects with hyaline cartilage. The fibrocartilage, mostly type I, does not seem very durable.194 A review of osteochondral fractures in humans reveals that the subchondral bone healing was with fibrous tissue only, and articular chondrocytes seem to contribute significantly to the repair response.224,231
Donohue formed an experimental model in which a canine patella was subjected to a stress of 25 newtons/mm2.199 He noted changes similar to those in osteoarthritis in the zone of calcified cartilage associated with a decrease in proteoglycans.195,199 In addition, there was an increase in prostaglandin synthesis after this blunt trauma. Hypothetically, a mechanism for matrix destruction would include an injury to the cell membrane releasing arachidonic acid, a precursor to prostaglandin E, which degrades the proteoglycan matrix with subsequent cell destruction.
Articular cartilage of the knee is exposed to acute and repetitive trauma from both endogenous and exogenous sources. The triaxial motion of the tibial-femoral articulation and the angle of inclination of the patella as it contacts its femoral sulcus make this joint susceptible to muscular imbalance and restriction of one motion as it relates to the other. A subsequent abnormal motion can result in a shearing force that can injure the superficial zone of the articular cartilage. Once this zone has been damaged, subsequent progression may occur. Also, recent reports have revealed MR images demonstrating alterations in the subchondral bone after joint trauma that could not be identified on conventional radiographs.240 These subchondral changes may lead to degenerative osteoarthritis240 or, as recently reported, may heal with restoration of normal articular cartilage and subchondral bone.241 Clinically, numerous acute knee injuries are being evaluated with MRIs that in some cases reveal a 'bone bruise.' Graf and associates204 found no correlation between the presence or location of a bone bruise with articular alterations at the time of arthroscopic surgery.
Over the years, several classification systems have been developed in an attempt to describe and categorize chondral injuries.189 With the advent of arthroscopy, it is preferable to use a classification system that is simple and reproducible. Noyes and Stabler have devised a system based on four separate variables: the description of the articular surface, the depth of involvement, the diameter of the lesion, and the location of the lesion (Table 29-7).226 This system is based on visual observations and contains a subjective element, but it does avoid terminology that is confusing and has different connotations for different observations.
Another classification system has been devised by Bauer and Jackson who identified six types of lesions of the articular cartilage. In their series, they found that rotational forces in direct trauma were the most common causes of injury to the articular cartilage. In most of their cases, the lesions were in the weight-bearing area of the articular cartilage, usually in the medial compartment. The most common associated lesion was a tear of the meniscus. The six types of changes are linear crack, stellate fracture, flap, crater, fibrillation, and degrading (Fig. 29-58). The linear crack is usually split-thickness, and it is often encountered on the lateral tibial plateau in association with ACL injuries. Stellate fracture is usually of a diverging type with central flaking of the cartilage. This is the most common type identified in the series (Fig. 29-59). In a flap tear, the cartilage is avulsed from the subchondral bone (Fig. 29-60). The crater is usually full-thickness and subchondral bone is exposed. Fibrillation is of partial thickness and corresponds to the old Outerbridge classification type III. The final characteristic appearance of abnormal articular cartilage is degenerative and extends down to the subchondral bone.
TABLE 29-7
Noyes' Classification of Articular Cartilage Lesions
Surface Description Extent of Involvement Diameter (mm) Location Degree of Knee Flexion
Cartilage surface intact A. Definite softening with <10 Patella Degree of Knee flexion
some resilience remaining </=15 A.proximal 1/3 where the lesion is in
middle 1/3 weight- bearing contact
Cartilage surface B. Extensive softening </=20 distal 1/3 (eg, 20°-45°)
damaged: cracks, with loss of resilience </=25 B.odd facet
fissures, fibrillation, or (deformation) >25 middle facet
fragmentation lateral facet
Bone exposed
A. < 1/2 thickness
A. Bone surface intact Medial femoral condyle
B. Bone surface a. anterior 1/3
cavitation b. middle 1/3
c. posterior 1/3
Lateral femoral condyle
a. anterior 1/3
b. middle 1/3
c. posterior 1/3
Medial tibial condyle
a. anterior 1/3
b. middle 1/3
c. posterior 1/3
Lateral tibial condyle
a. anterior 1/3
b. middle 1/3
c. posterior 1/3
(Noyes, F., and Stabler, C.L.: A system for Grading Articular Cartilage Lesions of Arthroscopy. Am. J. of Sports Med. 17(4): 505-513, 1989.)
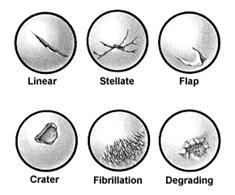
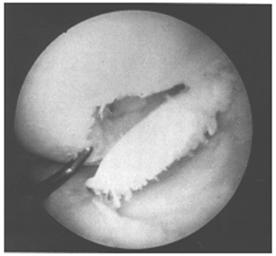
Fig. 28-60
Chondral injuries are difficult to diagnose as they mimic meniscal and synovial mishaps. Symptoms are nonspecific and often are associated with an effusion. When immediate, swelling usually implies associated vascular injury, whether it be subchondral bone or synovium. Delayed swelling is more likely to indicate a synovial response and usually occurs within 24 hours of injury. Pain can be either localized or diffuse. Diffuse pain is probably secondary to subchondral overload due to destruction of the overlying buffer layer (articular cartilage). Intermittent locking, recurrent effusions, crepitus, and persistent pain may all be associated with chondral injuries; this can present a diagnostic dilemma, as extensor mechanism dysfunction and meniscal injuries can present similarly.
When the chondral injury is associated with subchondral bone involvement, the subsequent radiopaque injury, a loose body, will be readily apparent on standard roentgenograms. It is important, however, to get more than just anteroposterior and lateral views (Fig. 29-61). In patients in the second to fourth decades of life, who are most susceptible to these types of injuries, tangential patella (Fig. 29-62A) and tunnel views should be obtained. Tunnel views are particularly important in patients susceptible to osteochondritis dissecans (Fig. 29-63). This lesion, most often seen on the lateral aspect of the medial femoral condyle, is usually unilateral (74%) and is twice as common in males.187,188 The age of affected persons ranges from 6 to 53, but the classical presentation occurs in those under age 18. Proposed etiologies are diffuse, and radiographically one must be careful not to confuse this with a normal growth plate.212 Similarly, osteochondritis dissecans of the patella is less common and might be mistaken for an osteochondral fracture of the patella (see Fig. 29-37).200,205,216 Avulsion injuries at the inferior pole of the patella216,223,231 also must not be confused with osteochondral fractures of the patella.
Arthrography has been used in the past to delineate big osteochondritis lesions, but it has not been helpful in small lesions. CT has also been considered. MRI has the added advantage of not exposing the patient to ionized radiation (Fig. 29-64). At present, however, it seems insensitive to defects of less than 3 mm.207 In fact, MRI's sensitivity to the early changes of chondral lesions has been reported to be low.227 However, it can delineate softening or thickening of the articular cartilage that could not be detected arthroscopically. In addition, MR images can demonstrate injury to the subchondral bone.204 Therefore, an MRI, as well as an arthroscopy, should be performed to evaluate a chondral injury fully. Arthroscopy remains the most accurate method of detailing the chondral defect, whether traumatic or osteochondrotic in nature. The invasive nature of arthroscopy, however, must be appreciated as both diagnostic and, in certain situations, therapeutic in nature.
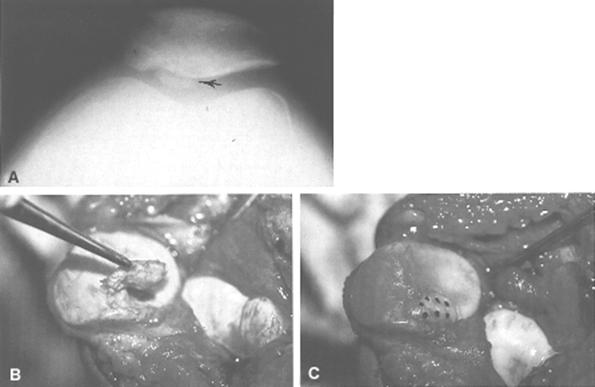
Fig.28-62 A) Fractura osteocondrala
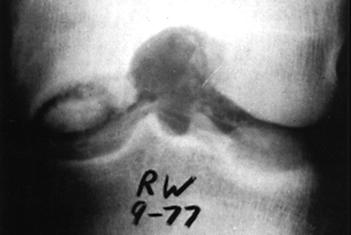
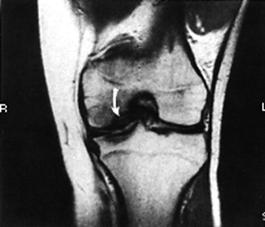
Fig. 28-64
Diagnosis of a chondral fracture can be made either indirectly by exclusion or through a diagnostic arthroscopy. Assuming meniscal and ligament injuries have been eliminated in the symptom complex, a diagnostic arthroscopy is often warranted. Diagnosis, however, should always be combined with a therapeutic approach and the patient appropriately warned.
Loose pieces of articular cartilage greater than 5 mm should be removed. Smaller pieces of articular cartilage often embed in the synovium and become asymptomatic. It is no longer appropriate to consider open arthrotomy. Because loose bodies can be removed through standard arthroscopic procedures,214 the trauma associated with this type of arthroscopic surgery is minimal and one should not hesitate in removing loose pieces of articular cartilage appropriately. With the multiple-portal technique, the loose piece(s) of articular cartilage can be identified and removed. This allows for identification of the site of origin, delineating the extent of the chondral fracture. Trimming the localized lesions has been beneficial in most series.211 Debridement by itself, however, which is usually confined to the arthritic population, is less predictable, alleviating symptoms for 75% of patients in short-term follow-ups.236 Theoretically, lavage of the knee during the arthroscopic procedure probably removes or diminishes the concentration of hydrolytic enzymes and the potential deleterious effects on the ground substance. Any reparative process would be dependent on stimulating the subchondral bone to subsequent formation of fibrin clots, as outlined previously. This, of course, would require drill holes in the manner initially described by Pridie.229 One must be careful not to injure articular cartilage during the arthroscopy procedure. To date, there has been no documented injury leading to subsequent arthritis, but it is always a concern, and follow-up time is still short.
Open reduction and internal fixation of osteochondral fractures have been attempted with Kirschner wires and screws. Long-term results with these treatments do not favor either technique; in fact, several large series report good results after removal of the osteochondral fragment.186,189,215,228,235,237 In general, small fragments need not be replaced, but when there are large fragments composing at least 25% of the joint surface area, consideration must be given to replacing the fracture fragment. In these cases, care should be taken that any internal fixation device does not interfere with the tibial and femoral articulation. In addition to the Herbert screw biodegradable pins (Orthosorb) and fibrin adhesive material have recently been introduced to promote fixation and healing of these fragments (see Fig. 29-62B and C). Experience to date is insufficient to formulate any definitive opinions.242
Postoperatively, there should be no joint immobilization because range of motion is encouraged. With osteochondrotic lesions, there is always some question as to when weight bearing is allowed. To date, there has been no evidence to favor non-weight bearing for more than 6 weeks.
Cartilage transplantation with autografts or allografts is becoming more accepted, although it is associated with significant problems. Donor matching, rejection of the graft, grafting incongruity, prolonged non-weight bearing, and early knee motion present formidable problems at this stage. The best results in several series showed 75% success at 4 years in patients with traumatic injuries.191
Recently, Brittberg and coworkers190 reported on the treatment of full-thickness defects of articular cartilage in the knee with autologous chondrocyte transplantation. This technique involves arthroscopically harvesting healthy chondrocytes from an uninvolved area of the injured knee. These chondrocytes are then cultured in the laboratory for 14 to 21 days. This is followed by a second surgical procedure in which an arthrotomy is performed and the chondral lesion is excised to normal surrounding articular cartilage. The defect is then covered with a periosteal flap, which is sutured in place. The cultured chondrocytes are then injected beneath the periosteal flap. After routine wound closure, active range-of-motion exercises and non-weight bearing are initiated. By about 8 weeks, patients are allowed full weight bearing. These investigators reported that 2 years after transplantation, 14 of 16 patients with femoral condylar transplants had good or excellent results; at an average follow-up of 3 years, 2 of 7 patellar transplants were good or excellent, 3 were fair, and 2 were poor. Biopsy of the transplant site demonstrated that 11 of the 15 femoral transplants and 1 of the 7 patellar transplants had the appearance of hyaline-like cartilage containing type II collagen. The results of this new technique appear encouraging, and further investigation must be undertaken.
Authors' Preferred Method of Treatment
In the acute setting for the patient in whom a diagnosis of chondral fracture is present after other diagnostic evaluations (eg, clinical examination, roentgenograms, MRI) are normal and the history suggests a nonligamentous chondral injury, our initial approach is symptomatic relief with devices that relieve weight bearing, ice, nonsteroidal anti-inflammatory medication, and encouragement of persistent range-ofmotion exercise. Swimming is one of the best modalities for maintaining this range of motion, especially if the patient avoids 'whip-kicks.' Quadriceps- and hamstring-strengthening exercises should be initiated, avoiding contact areas through the range of motion at the site of the articular cartilage defect.
If discomfort and swelling persist for more than 6 weeks after injury, it is reasonable to consider arthroscopic evaluation, possible removal of loose articular cartilage, and debridement of degenerative cartilage. Although symptoms of discomfort can vary, swelling suggests an ongoing process that, as noted, might be injurious to the extracellular matrix. The risk-benefit ratio for arthroscopic diagnosis and treatment warrants serious consideration when a patient's symptoms persist.
We do not replace pieces of articular cartilage unless they are acute, large osteochondral fractures that are amenable to fixation. There is no rule as to what size fragment should be removed, but with a fragment in excess of 25% of the joint surface, we often attempt to reposition it (see Fig. 29-62).
|
Politica de confidentialitate |
| Copyright ©
2025 - Toate drepturile rezervate. Toate documentele au caracter informativ cu scop educational. |
Personaje din literatura |
| Baltagul – caracterizarea personajelor |
| Caracterizare Alexandru Lapusneanul |
| Caracterizarea lui Gavilescu |
| Caracterizarea personajelor negative din basmul |
Tehnica si mecanica |
| Cuplaje - definitii. notatii. exemple. repere istorice. |
| Actionare macara |
| Reprezentarea si cotarea filetelor |
Geografie |
| Turismul pe terra |
| Vulcanii Și mediul |
| Padurile pe terra si industrializarea lemnului |
| Termeni si conditii |
| Contact |
| Creeaza si tu |