Silver bromide (AgBr), a soft, pale-yellow, water insoluble salt well known (along with other silver halides) for its unusual sensitivity to light . Bromura de argint (AgBr), o moale, galben-pal, sare de apa insolubil bine cunoscut (impreuna cu alte halogenuri de argint), pentru sensibilitatea sa neobisnuite la lumina. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for faking the Shroud of Turin . The salt can be found naturally as the mineral bromargyrite (bromyrite). Aceasta proprietate a permis halogenuri de argint pentru a deveni baza moderne materiale fotografice. AgBr este utilizat pe scara larga in filme fotografice si se crede de unii a fi fost folosite pentru preface Giulgiul din Torino. de sare poate fi gasit in mod natural ca bromargyrite minerale (bromyrite).
Although the compound can be found in mineral form, AgBr is typically prepared by the reaction of silver nitrate with an alkali bromide, typically potassium bromide: Desi compus poate fi gasit in forma minerale, AgBr este de obicei pregatita de reactie de nitrat de argint, cu o bromura de alcaline, de obicei bromura de potasiu:
AgNO 3 (aq) + KBr(aq) → AgBr(s)+ KNO 3 (aq) AgNO 3 (AQ) + KBr (AQ) → AgBr (s) + KNO 3 (AQ)
Although less convenient, the salt can be also prepared directly from its elementss Desi mai putin convenabil, de sare poate fi, de asemenea, pregatite direct de la elementss sale
Modern preparation of a simple, light-sensitive surface involves forming an emulsion of silver halide crystals in a gelatine, which is then coated on a film or other support. Moderne de pregatire a unui simplu, lumina-suprafata sensibila implica formarea unei emulsii de cristale de halogenuri de argint intr-o gelatina, care este apoi acoperita pe un film sau alte tipuri de sprijin. The crystals are formed by precipitation in a controlled environment to produce small, uniform crystals (typically < 1 μm in diameter and containing ~10 12 Ag atoms) called grains. Cristalele sunt formate prin precipitare intr-un mediu controlat pentru a produce mici, cristale uniforme (de obicei, <1 μm in diametru si cu continut de ~ 10 12 atomi de Ag), numit boabe.
Silver bromide reacts readily with liquid ammonia to generate a variety of amine complexes: [ 3 ] Bromura de argint reactioneaza rapid cu amoniac lichid pentru a genera o varietate de complexe amine:
AgBr + nNH 3 → Ag(NH 3 ) 2 1+ AgBr + nNH 3 → Ag (NH 3) 2 1 +
(AgBr (NH 3) 2)
(AgBr 2 (NH 3) 2) 1 --
(AgBr (NH 3))
(AgBr 2 (NH 3)) 1 --
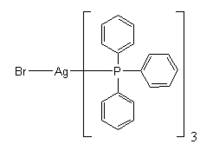
Silver bromide reacts with triphenylphosphine to give a tris(triphenylphosphine) product: Bromura de argint reactioneaza cu triphenylphosphine pentru a da un tris (triphenylphosphine) produs:
AgF, AgCl, and AgBr all have face-centered cubic (fcc) rock-salt (NaCl) lattice structure with the following lattice parameters: [ 5 ] AGF, AgCl, si toate au AgBr fata-centrat cubi (FCC) rock-sare (NaCl), grilaje de structura cu parametrii Lattice dupa cum urmeaza: [5]
The larger halide ions are arranged in a cubic close-packing, while the smaller silver ions fill the octahedral gaps between them, giving a 6-coordinate structure where a silver ion Ag + is surrounded by 6 Br − ions and vice-versa. Ionii mai mari cu halogenuri sunt aranjate intr-o stransa cubi de ambalare, in timp ce mai mici, ionii de argint umple golurile octaedrale intre ele, dand o 6-a coordona cazul in care o structura de ioni de argint AG + este inconjurat de 6 Br - ioni si vice-versa. The coordination geometry for AgBr in the NaCl structure is unexpected for Ag(I) which typically forms linear, trigonal (3-coordinated Ag) or tetrahedral (4-coordinated Ag) complexes. Geometrie de coordonare pentru AgBr in structura NaCl este neasteptata pentru Ag (I), care de obicei forme liniare, trigonal (3-coordonate Ag) sau tetrahedral (4-coordonate Ag) complexe.
Unlike the other silver halides, iodargyrite (AgI) contains a hexagonal zincite lattice structure. Spre deosebire de alte halogenuri de argint, iodargyrite (AGI) contine o structura hexagonala zincite zabrele.
The silver halides have a wide range of solubilities, noting that the solubility of AgF is about 6 × 10 7 times greater than that of AgI. Halogenuri de argint au o gama larga de solubilitati, constatand ca solubilitatea AGF este de aproximativ 6 × 10 7 ori mai mare decat cea a AGI. These differences are attributed to the relative solvation enthalpies of the halide ions; the enthalpy of solvation of fluoride is anomalously large. Aceste diferente sunt atribuite enthalpies relativa solvatare a ionilor halogenuri; entalpia de solvatare a fluorura este anomalously mare.
|
Silver halide solubilities Solubilitati halogenuri de argint |
|
|
Compuse |
Solubilitate (g / 100 g H 2 O) |
|
AgF | |
|
AgCl | |
|
AgBr | |
|
AgI | |
Although photographic processes have been in development since the mid 1800's, there were no suitable theoretical explanations until 1938 with the publication of a paper by RW Gurney and NF Mott. [ 7 ] This paper triggered a large amount of research in fields of solid-state chemistry and physics, as well more specifically in silver halide photosensitivity phenomena. Cu toate ca procesele de fotografice au fost in dezvoltare din mijlocul anilor 1800, nu au existat explicatii adecvate teoretice pana in 1938 cu publicarea unui document de RW Gurney si NF Mott. Aceasta lucrare a declansat o mare cantitate de cercetare in domeniile de solid-state chimie si fizica, precum si in mod special in mai mult de argint fenomene de fotosensibilitate halogenuri.
Moreover, further research into this mechanism revealed that the photographic properties of silver halides (in particular AgBr) were a result of deviations from an ideal crystal structure. Mai mult decat atat, in continuare cercetari in acest mecanism a aratat ca proprietatile fotografice de halogenuri de argint (in special AgBr) au fost urmare a abaterilor de la o structura de cristal ideal. Factors such as crystal growth, impurities, and surface defects all contribute to affect concentrations of point ionic defects and electronic traps, which subsequently affect the sensitivity to light and allow for the formation of a latent image . Factori cum ar fi cresterea de cristal, impuritati, si defectele de suprafata toate contribuie la afecteze concentratiile de la punctul defecte ionice si capcane electronice, care ulterior afecteaza sensibilitate la lumina si sa permita formarea unei imagini latente.
Frenkel defects and quadropolar deformation Defecte Frenkel si quadropolar de deformare
The major defect in silver halides is the Frenkel defect , where silver ions are located interstitially (Ag i + ) in high concentration with their corresponding negatively-charged silver ion vacancies (Ag v − ). Defect major in halogenuri de argint este defect Frenkel, in cazul in care ionii de argint sunt situate interstitially (Ag i +) in concentratie ridicata cu posturile vacante corespunzatoare acestora negativ perceput-Ion de argint (AG / -). What is unique about AgBr Frenkel pairs is that the interstitial Ag i + are exceptionally mobile, and that its concentration in the layer below the grain surface (called the space charge layer) far exceeds that of the intrinsic bulk. The formation energy of the Frenkel pair is low at 1.16 eV, and the migration activation energy is unusually low at 0.05 eV (compare to NaCl: 2.18 eV for the formation of a Schottky pair and 0.75 eV for cationic migration). Ce este unic la AgBr perechi Frenkel este ca interstitiale Ag i + sunt in mod exceptional de telefonie mobila, si ca, concentratia acesteia in stratul de sub suprafata de cereale (numita taxa de spatiu strat) depaseste cu mult cea a vrac intrinseci. energiei formarea pereche Frenkel este redus de la 1.16 eV, energia si migratia de activare este neobisnuit de scazut la 0.05 eV (a se compara cu NaCl: 2.18 eV pentru formarea unei perechi Schottky si 0,75 eV de migratie cationice). These low energies result in large defect concentrations, which can reach near 1% near the melting point. Aceste rezultat scazut de energie in concentratii defect mari, care pot ajunge la aproape 1% in apropierea punctului de topire.
The cause of the low activation energy in silver bromide can be attributed the silver ions' high quadrupolar polarizability; that is, it can easily deform from a sphere into an ellipsoid. Veninos de la o energie de activare de scazute in bromura de argint poate fi atribuita de mare ionii de argint 'polarizability quadrupolar, ca este, poate deforma cu usurinta de la o sfera intr-o elipsoidala. This property, a result of the d 9 electronic configuration of the silver ion, facilitates migration in both the silver ion and in silver ion vacancies, thus giving the unusually low migration energy (for Ag v − : 0.29-0.33 eV, compared to 0.65 eV for NaCl). Aceasta proprietate, un rezultat al d 9 configuratia electronica a ioni de argint, faciliteaza migratia atat in ioni de argint si in posturile vacante de ioni de argint, dand astfel de energie neobisnuit de scazut de migrare (pentru AG / -: 0.29-0.33 eV, in comparatie cu 0.65 eV pentru NaCl).
Studies have demonstrated that the defect concentrations are strongly affected (up to several powers of 10) by crystal size. Studiile au demonstrat ca concentratiile defectul sunt puternic afectate (pana la mai multe puteri de 10) in functie de dimensiunea de cristal. Most defects, such as interstitial silver ion concentration and surface kinks are inversely proportional to crystal size, although vacancy defects are directly proportional. Cele mai multe defecte, cum ar fi interstitiala concentratie de ioni de argint si de kinks de suprafata sunt invers proportionala cu marimea de cristal, cu toate ca defectele post vacant sunt direct proportionale. This phenomenon is attributed to changes in the surface chemistry equilibrium, and thus affects each defect concentration differently. Acest fenomen este atribuita la schimbari de echilibru suprafata chimie, si, astfel, afecteaza in fiecare concentratie de defect de diferit.
Impurity concentrations can be controlled by crystal growth or direct addition of impurities to the crystal solutions. Concentratiile impuritate pot fi controlate de crestere de cristal sau adaugarea directa a impuritatilor la solutii de cristal. Although impurities in the silver bromide lattice are necessary to encourage Frenkel defect formation, studies by Hamilton have shown that after a particular concentration of impurities, the number of defects of interstitial silver ions and positive kinks reduce sharply by several orders of magnitude. Cu toate impuritatile din grilaj de bromura de argint sunt necesare pentru a incuraja Frenkel formarea defect, studii de Hamilton au aratat ca, dupa o concentrare a impuritatilor, numarul de defecte de ioni de argint interstitiala si kinks pozitiva a reduce brusc de cateva ordine de marime. After this point, only silver ion vacancy defects, which actually increase by several orders of magnitude, are prominent. Dupa acest moment, numai defecte de ioni de argint posturile vacante, care de fapt creste cu cateva ordine de marime, sunt proeminente.
Electron traps and hole traps Capcane Electron si capcane gaura
When light is incident on the silver halide grain surface, a photoelectron is generated when a halide loses its electron to the conduction band: Cand lumina este incidentul de pe suprafata de argint halogenuri de cereale, un fotoelectron este generat atunci cand un halogenuri isi pierde electroni sa de a benzii de conducta:
X - + hν → X + e - X - + hν → X + e --
After the electron is released, it will combine with an interstitial Ag i + to create a silver metal atom Ag i 0 : Dupa ce electronul este eliberata, ea se va combina cu o interstitiale Ag i + pentru a crea un atom de metal argint Ag I 0:
e − + Ag i + → Ag i 0 e - + Ag i + → Ag I 0
Through the defects in the crystal the electron is able to reduce its energy and become trapped in the atom. The extent of grain boundaries and defects in the crystal affect the lifetime of the photoelectron, where crystals with a large concentration of defects will trap an electron much faster than a purer crystal. Prin defecte in cristal electronul este in masura de a reduce consumul de energie si sa devina prinse in atomului. gradul de granitele de cereale si defecte de cristal afecteaza durata de viata a fotoelectron, in cazul in care cristalele cu o concentratie mare de defecte vor capcana un electron mult mai repede decat un cristal mai pure.
When a photoelectron is mobilized a photohole h. is also formed, which, too, needs to be neutralized. Atunci cand o fotoelectron este mobilizat un photohole . h este, de asemenea, format, care, de asemenea, trebuie sa fie neutralizate. The lifetime of a photohole, however, does not correlate with that of a photoelectron. Pe durata de viata a unui photohole, cu toate acestea, nu se coreleaza cu cea a unui fotoelectron. This detail suggests a different trapping mechanism; Malinowski suggests that the hole traps may be related to defects as a result of impurities. Once trapped, the holes attract mobile, negatively-charged defects in the lattice: the interstitial silver vacancy Ag v − : Acest detaliu sugereaza un mecanism diferit de vanatoare cu capcane; Malinowski sugereaza ca capcanele gaura pot fi legate de defecte, ca urmare a impuritatilor. Odata prins, orificiile atrage defecte negativ perceput-mobile, in grilaj: post vacant interstitiale argint AG / -:
h. + Ag v
− . h + AG / -- ![]() h.Ag v
h.Ag v
h.Ag v
h.Ag v
The formation of the h.Ag v lowers its energy
sufficiently to stabilize the complex and reduce the probability of ejection of
the hole back into the valance band (the equilibrium constant for hole-complex
in the interior of the crystal is estimated at 10 −4 . Formarea v
h.Ag de energie scade suficient pentru a stabiliza complexe si de a reduce
probabilitatea de ejectie a gaura inapoi in banda de Valance (echilibru
Additional investigations on electron- and hole-trapping
demonstrated that impurities also can be a significant trapping system.
Investigatii suplimentare privind electron-si gaura-capcana a
demonstrat ca impuritati, de asemenea, poate fi un sistem de
semnificativ de vanatoare cu capcane. Consequently,
interstitial silver ions may not be reduced. Prin urmare, ionii de
argint interstitiala nu poate fi redus. Therefore,
these traps are actually oss mechanisms and are considered trapping
inefficiencies. Mecanismele Prin urmare, aceste capcane sunt de fapt
Crystal surface chemistry;
Once the hole-complexes are formed, they diffuse to the surface of the grain as a result of the formed concentration gradient. Odata ce gaura-complexe sunt formate, acestea difuze pe suprafata de cereale, ca urmare a gradientului de concentrare format. Studies demonstrated that the lifetime of holes near the surface of the grain are much longer than those in the bulk and that these holes are in equilibrium with adsorbed bromine. Studiile au demonstrat ca durata de viata a gaurilor in apropierea suprafetei de cereale sunt mult mai lungi decat cele in vrac si ca aceste gauri sunt in echilibru cu brom adsorbit. The net effect is an equilibrium push at the surface to form more holes. Efectul net este o impinge de echilibru la suprafata, pentru a forma gauri mai mult. Therefore, as the hole-complexes reach the surface, they disassociate: Prin urmare, dupa cum gaura-complexele ajunge la suprafata, acestea disocieze:
h.Ag v − → h. + Ag v − → Br → ½ Br 2 v h.Ag - → . h + AG / - → Br → ½ Br 2
By this reaction equilibrium, the hole-complexes are constantly consumed at the surface, which acts as a sink, until removed from the crystal. Prin acest echilibru reactie, gaura-complexele sunt consumate in mod constant la suprafata, care actioneaza ca o chiuveta, pana la scos din cristal. This mechanism provides the counterpart to the reduction of the interstitial Ag i + to Ag i 0 , giving an overall equation of: Acest mecanism prevede o contrapartida la reducerea interstitiale Ag i + la Ag I 0, oferind o ecuatie de ansamblu a:
AgBr → Ag + ½ Br 2 AgBr → Ag + ½ Br 2
Latent image formation and photography Latent formarea imaginii si fotografie
Now that some of the theory has been presented, the actual mechanism of the photographic process can now be discussed. Acum, ca unele dintre teorie a fost prezentat, mecanismul real al procesului de fotografic poate fi acum discutat. To summarize, as a photographic film is subjected to an image, photons incident on the grain produce electrons which interact to yield silver metal. Pentru a rezuma, ca un film fotografic este supus la o imagine, fotoni incident de pe cereale produc electronii care interactioneaza pentru a produce metal de argint. More photons hitting a particular grain will produce a larger concentration of silver atoms, containing between 5 and 50 silver atoms (out of ~10 12 atoms), depending on the sensitivity of the emulsion. Fotoni Mai multe lovind un bob special, va produce o concentrare mai mare de atomi de argint, care contin intre 5 si 50 atomi de argint (din ~ 10 12 atomi), in functie de sensibilitatea emulsie. The film now has a concentration gradient of silver atom specks based upon varying intensity light across its area, producing an invisible ' latent image '. Filmul are acum pe o panta de concentrare a petelor atom de argint, pe baza variind intensitatea luminii in zona sa, care produc un invizibil 'imagine latenta'.
While this process is occurring, bromine atoms are being produced at the surface of the crystal. In timp ce acest proces se produce, atomi de brom sunt produse la suprafata de cristal. To collect the bromine, a layer on top of the emulsion, called a sensitizer, acts as a bromine acceptor. Pentru a colecta brom, un strat pe partea de sus a emulsie, numit sensitizer, actioneaza ca un acceptor de brom.
During film development the latent image is intensified by addition of a chemical, typically hydroquinone, that selectivity reduces those grains which contain atoms of silver. In timpul de dezvoltare de film imaginea latenta este intensificat prin adaugarea unui produs chimic, de obicei de hidrochinona, ca selectivitatea reduce aceste cereale care contin atomi de argint. The process, which is sensitive to temperature and concentration, will completely reduce grains to silver metal, intensifying the latent image on the order of 10 10 to 10 11 . Procesului, care este sensibil la temperatura si de concentrare, va reduce complet boabe de la metal de argint, intensificarea imaginea latenta pe ordinea de 10 10 la 10 11. This step demonstrates the advantage and superiority of silver halides over other systems: the latent image, which takes only milliseconds to form and is invisible, is sufficient to produce a full image from it. Acest pas demonstreaza avantajele si superioritatea halogenuri de argint peste alte sisteme: imagine latenta, care tine doar milisecunde, pentru a forma si este invizibil, este suficienta pentru a produce o imagine completa de la ea.
After development, the film is 'fixed,' during which the remaining silver salts are removed to prevent further reduction, leaving the 'negative' of the film. Dupa dezvoltare, filmul este 'fixe', in care restul de sarurile de argint sunt indepartate pentru a preveni reducerea in continuare, lasand negativ ',' din film. The agent used is sodium thiosulphate, and reactions according to the following equation: Agentul folosit este tiosulfat de sodiu, precum si reactii in conformitate cu urmatoarea ecuatie:
AgX(s) + 2 Na 2 S 2 O 3 (aq) → Na 3 [Ag(S 2 O 3 ) 2 ](aq) + NaX(aq) AGX (s) + 2 Na 2 S 2 O 3 (AQ) → Na 3 [Ag (S 2 O 3) 2] (AQ) + NaX (AQ)
An indefinite number of positive prints can be generated from the negative by passing light through it and undergoing the same steps outlined above. Un numar nedeterminat de amprente pozitive pot fi generate de negative prin care trece lumina prin el si in curs de aceiasi pasi prezentati mai sus.
As silver bromide is heated within 100 °C of its melting point, an Arrhenius plot of the ionic conductivity shows the value increasing and 'upward-turning.' Dupa cum bromura de argint este incalzita in termen de 100 ° C din punct de topire sale, un complot Arrhenius de conductivitatea ionica arata valoarea in crestere si 'sus-cotitura.' Other physical properties such as elastic moduli, specific heat, and the electronic energy gap also increase, suggesting the crystal is approaching instability. This behavior, typical of a semi-conductor, is attributed to a temperature-dependence of Frenkel defect formation, and, when normalized against the concentration of Frenkel defects, the Arrhenius plot linearizes. Alte proprietati fizice, cum ar fi moduli elastice, caldura specifica, iar diferenta de electronice de energie, de asemenea, creste, sugerand cristal se apropie de instabilitate. Acest comportament, tipica a unui semi-conductor, este atribuita la o temperatura-dependenta de formare a defectului Frenkel , si, atunci cand normalizat impotriva concentratia de defecte Frenkel, linearizes Arrhenius parcelei.
|
Politica de confidentialitate |
| Copyright ©
2025 - Toate drepturile rezervate. Toate documentele au caracter informativ cu scop educational. |
Personaje din literatura |
| Baltagul – caracterizarea personajelor |
| Caracterizare Alexandru Lapusneanul |
| Caracterizarea lui Gavilescu |
| Caracterizarea personajelor negative din basmul |
Tehnica si mecanica |
| Cuplaje - definitii. notatii. exemple. repere istorice. |
| Actionare macara |
| Reprezentarea si cotarea filetelor |
Geografie |
| Turismul pe terra |
| Vulcanii Și mediul |
| Padurile pe terra si industrializarea lemnului |
| Termeni si conditii |
| Contact |
| Creeaza si tu |